INSPIRE: AN ALTERNATIVE TREATMENT FOR OBSTRUCTIVE SLEEP APNEA
- Posted on: Mar 21 2023
 If you have obstructive sleep apnea (OSA) you are likely familiar with CPAP—a machine that delivers pressurized air through a mask worn while you sleep to keep the airway open. While CPAP is an effective solution to treat OSA, it is not always the right choice for everyone. If you have tried CPAP but still struggle with obstructive sleep apnea, Inspire may be the right treatment option for you.
If you have obstructive sleep apnea (OSA) you are likely familiar with CPAP—a machine that delivers pressurized air through a mask worn while you sleep to keep the airway open. While CPAP is an effective solution to treat OSA, it is not always the right choice for everyone. If you have tried CPAP but still struggle with obstructive sleep apnea, Inspire may be the right treatment option for you.
What is Inspire?
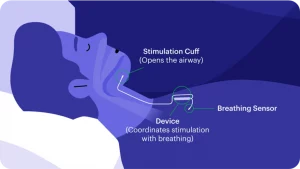
Inspire in an FDA approved implantable device designed to open the airway during sleep to decrease obstructive sleep apnea. Inspire works by stimulating the nerve that controls the tongue to gently move it forward and out of the airway. The user has control over the device using a handheld remote. Before going to bed the patient will turn on the device and then turn it off when waking in the morning. The device can also be paused, and settings can be changed using the remote.
Inspire consists of three components:
- A breathing sensor located in the chest wall
- A nerve stimulator that stiffens the tongue and moves it forward to open the airway placed in the upper neck
- A pulse generator that provides the power for the nerve stimulator, similar to a pacemaker, also located in the chest wall
After ensuring that all eligibility requirements are met, Inspire can be placed during an outpatient procedure. The procedure involves an incision in the upper chest wall to place the breathing sensor and the pulse generator, as well as an incision in the neck to place the nerve stimulator. The procedure generally takes 1-2 hours and patients are able to return home the same day as the procedure. Most patients only experience mild discomfort and swelling at the incision sites following the procedure.
Follow Up Care
The first step after the procedure is to allow time for the incisions to heal. This typically takes about 30 days, and the device is not turned on during this time.
Once you have finished healing you will be seen by a sleep medicine provider to have the device activated. When the device is activated you will have control of it using the handheld remote to increase the stimulation to the desired outcome which is for resolution of snoring and obstructive sleep apnea events. You will be asked to have a fine tuning titration of the stimulation level in a sleep lab about 60 days after activation to optimize the settings of the implant.
Who is a candidate for Inspire?
Ideal Inspire candidates are over 18 years of age and:
- Have moderate to severe obstructive sleep apnea (OSA)
- Have tried CPAP but cannot tolerate or do not benefit from therapy
- Meet certain BMI requirements (usually BMI less than 32)
If the above criteria are met, the patient is scheduled to undergo a drug induced sleep endoscopy (DISE). A DISE is an evaluation technique to observe how the throat moves during sleep.
The DISE is a quick non-surgical outpatient procedure that is performed in the office. During the procedure an anesthesiologist gives you medication to make you fall asleep. While you are asleep a small camera in inserted in the nose to examine the throat and parts of the airway that contribute to obstructive sleep apnea. A DISE is necessary to ensure that Inspire will be effective at controlling your OSA before implanting the device.
SCHEDULE A CONSULTATION
If you think you may be a candidate for Inspire, call to schedule an appointment at Sinus and Snoring Specialists for a thorough evaluation. Contact us today at 512-601-0303 to schedule an appointment.
Tagged with: Inspire, Inspire therapy, Sleep apnea, sleep apnea treatment
Posted in: Inspire Therapy, Sleep Apnea

